Abstract
Introduction
Outcomes for patients with relapsed and refractory multiple myeloma (RRMM) and previous exposure to immunomodulatory agents, proteasome inhibitors (PIs), and anti-CD38 antibodies are poor. Ide-cel is a B-cell maturation antigen (BCMA)-directed CAR T cell therapy. In the pivotal phase 2 KarMMa trial (NCT03361748), ide-cel demonstrated frequent, deep, and durable responses in heavily pretreated patients with RRMM (Munshi et al. N Engl J Med 2021). In the 42 patients (33%) who achieved compete response (CR) or stringent CR (sCR), median duration of response was 21.5 months (Anderson et al. ASCO 2021. Poster 8016). Currently it is difficult to predict which patients will achieve deep responses from ide-cel. To identify clinical correlates of patients achieving CR/sCR with ide-cel, this subanalysis of KarMMa compared baseline characteristics between patients with CR/sCR and non-CR/sCR.
Methods
Patients with MM, ≥ 3 prior lines of therapy (including an immunomodulatory agent, PI, and anti-CD38 antibody), and disease refractory to last regimen per IMWG criteria received ide-cel infusion (target dose range 150-450 x 10 6 CAR+ T cells) after lymphodepletion (fludarabine 30 mg/m 2/day + cyclophosphamide 300 mg/m 2/day for 3 days). Bridging therapy was optional (last dose ≥ 14 days prior to lymphodepletion). The primary endpoint was overall response rate; CR/sCR rate was a key secondary endpoint. Baseline characteristics were collected prior to lymphodepletion and for select biomarkers on day of infusion. In this subanalysis, univariate and multivariate logistic regression models were used to identify baseline characteristics that correlate with the likelihood of achieving CR/sCR.
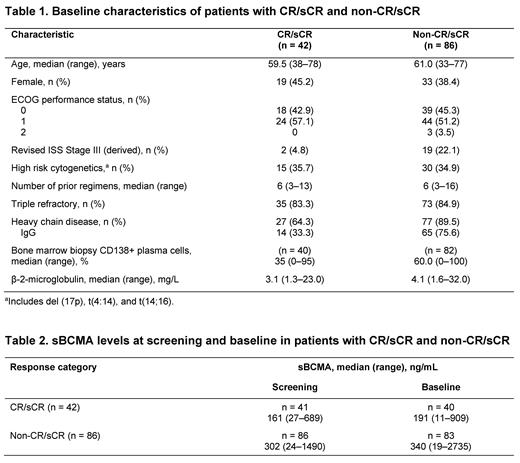
Results
In total, 128 of 140 patients received ide-cel infusions at data cutoff (Dec 21, 2020). There were 42 patients with best overall response of CR/sCR and 86 with non-CR/sCR (very good partial response [VGPR], partial response [PR], or no response). Among those with CR/sCR, 32 (76%) were negative for minimal residual disease (MRD) at a sensitivity level of < 10 -5 nucleated cells and 19 of these patients maintained MRD negativity at the 12-month follow-up. Baseline demographics and disease characteristics were generally balanced between patients with CR/sCR and non-CR/sCR; notable exceptions included revised International Staging System (ISS) stage III disease, IgG chain type, CD138+ plasma cell percentage, and β-2-microglobulin levels (Table 1). Univariate analysis of CR/sCR by baseline characteristics showed that IgG heavy chain versus other heavy chain types (odds ratio [OR]: 0.162, P < 0.0001), high sBCMA (OR: 0.646, P = 0.0007), β-2-microglobulin (≥ 5.5 vs < 3.5 mg/L; OR: 0.201, P = 0.0072), and presence of extramedullary disease (OR: 0.428, P = 0.0394) were negatively associated with CR/sCR, whereas high vector copy number in drug product was positively associated with CR/sCR (OR: 1.290, P = 0.0287). A multivariate analysis of CR/sCR identified IgG heavy chain versus other heavy chain types (OR: 0.100, P < 0.0001), high sBCMA (OR: 0.637, P = 0.0110), and elevated prothrombin time-international normalized test (OR: 0.005, P = 0.0365) as negative correlates of CR/sCR, and high vector copy number in drug product (OR: 1.486, P = 0.0168) as a positive correlate of CR/sCR. Descriptive analysis demonstrated lower baseline sBCMA in patients with CR/sCR versus non-CR/sCR; across both groups, sBCMA levels increased between screening and baseline (Table 2).
Conclusions
In this subanalysis of KarMMa, multivariate analysis identified IgG, sBCMA, and prothrombin time-international normalized test as negative correlates of CR/sCR, and vector copy number in drug product a positive correlate. As sBCMA is an indicator of tumor burden and can affect therapies targeting BCMA (Cowan et al. ASH 2019. Abstract 204), selecting for patients with lower tumor burden and controlling tumor burden during manufacturing or bridging therapy may be important in achieving CR/sCR with ide-cel. The impact of modulating tumor BCMA and sBCMA, using a gamma-secretase inhibitor prior to ide-cel infusion, is currently under evaluation in the KarMMa-7 trial.
Study support
bluebird bio and Celgene, a Bristol-Myers Squibb Company.
Shah: Indapta Therapeutics: Consultancy; Janssen: Research Funding; Sutro Biopharma: Research Funding; Poseida: Research Funding; Precision Biosciences: Research Funding; Amgen: Consultancy; Bluebird Bio: Research Funding; Teneobio: Research Funding; CareDx: Consultancy; GSK: Consultancy; BMS/Celgene: Research Funding; CSL Behring: Consultancy; Karyopharm: Consultancy; Sanofi: Consultancy; Kite: Consultancy; Nektar: Research Funding; Oncopeptides: Consultancy. Munshi: Abbvie: Consultancy; Pfizer: Consultancy; Janssen: Consultancy; Bristol-Myers Squibb: Consultancy; Amgen: Consultancy; Takeda: Consultancy; Oncopep: Consultancy, Current equity holder in publicly-traded company, Other: scientific founder, Patents & Royalties; Celgene: Consultancy; Karyopharm: Consultancy; Adaptive Biotechnology: Consultancy; Novartis: Consultancy; Legend: Consultancy. Berdeja: Lilly, Novartis: Research Funding; Poseida, Sanofi, Teva: Research Funding; GSK, Ichnos Sciences, Incyte: Research Funding; EMD Sorono, Genentech: Research Funding; Celularity, CRISPR Therapeutics: Research Funding; Bluebird bio, BMS, Celgene, CRISPR Therapeutics, Janssen, Kite Pharma, Legend Biotech, SecuraBio, Takeda: Consultancy; Abbvie, Acetylon, Amgen: Research Funding. Jagannath: Legend Biotech: Consultancy; Karyopharm Therapeutics: Consultancy; Janssen Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; Sanofi: Consultancy; Takeda: Consultancy. Finney: bluebird bio: Current Employment, Current equity holder in publicly-traded company. Martin: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Agarwal: Bristol Myers Squibb: Current Employment, Current holder of individual stocks in a privately-held company. Rowe: Bristol Myers Squibb: Current Employment. Campbell: Bristol Myers Squibb: Current Employment, Current holder of individual stocks in a privately-held company. San-Miguel: AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, Merck Sharpe & Dohme, Novartis, Regeneron, Roche, Sanofi, SecuraBio, Takeda: Consultancy, Other: Advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal